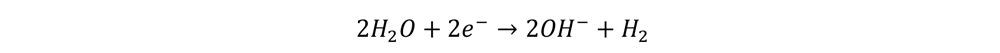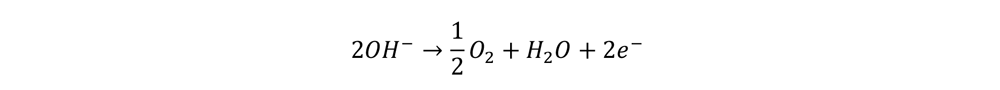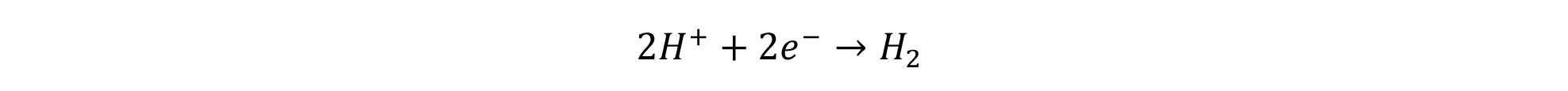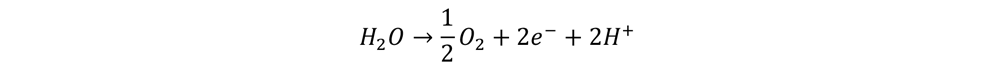ELECTROLYSIS HYDROGEN ELECTROLYSIS TECHNOLOGIES: A COMPARATIVE OVERVIEW Electrolysis is a key technology in the production of green hydrogen (H2), which is hydrogen generated from water (H2O) using electricity sourced from renewable energy. This elegant solution involves, submerging two electrodes in water and attaching these electrodes to a direct current source at a voltage > 1.23 V. Under these conditions electrochemical reactions involving electron transfer reactions occur at the surface of these electrodes. In pure water the reaction is slow, so in commercial electrolyzers a catalyst is added to speed up the reactions. So, the solution is either acidic or alkaline, that is because the addition of either H+ (acidic solution) or OH- (alkaline solution) act as catalysts, speeding up the reaction while not being used up in the overall reaction … which is
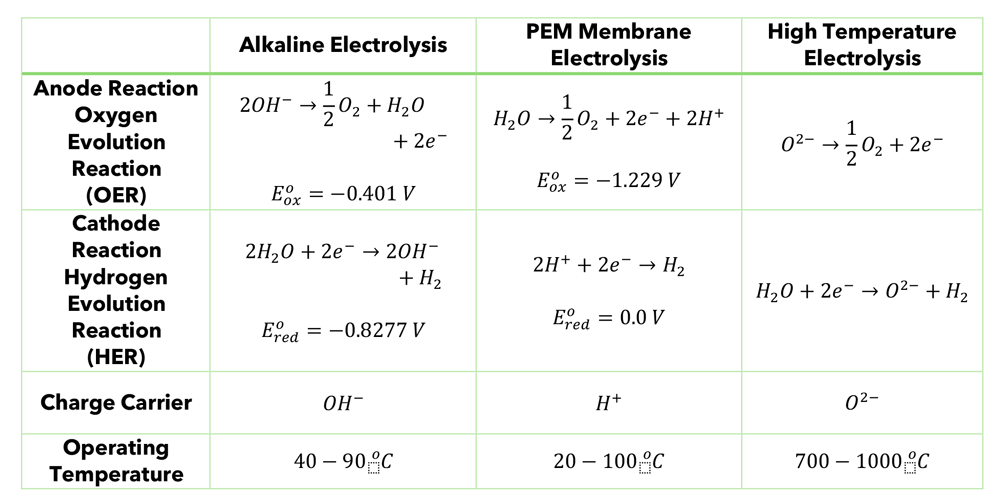
The electrode that is negatively charged (cathode) is where the Hydrogen Evolution Reaction (HER) occurs. BASE CATALYZED WATER ELECTROLYSIS (BCWE)In an alkaline medium this reaction is
The electrode that is positively charged (anode) attracts releases oxygen. In an alkaline medium the reaction that occurs is
Note that the hydroxide is created at the cathode and used up at the anode. If the solution is alkaline, it already contains appreciable concentrations of the hydroxide ion (OH-). This speeds up the reaction and does not get consumed overall, that is what we call a catalyst. So, water electrolysis in an alkaline medium is hydroxide catalyzed water electrolysis.
ACID CATALYZED WATER ELECTROLYSIS (ACWE)We can go the other way. Instead of performing the reaction in a basic (alkaline) medium, we can perform the reaction in an acidic environment. The reactions at the electrodes are slightly different.
At the cathode the reaction is …
While at the anode the reaction becomes …
Again, note that H+ is used up at the cathode and regenerated at the anode. It is therefore a catalyst.
Chemical reactions happen at certain rates that depend on conditions such as the temperature, concentration of the catalysts, and according to the simplicity of the reaction. Some reactions happen in multiple steps, and those can be slower as a consequence. In alkaline water electrolysis the oxygen reaction is the fastest, while in acidic water electrolysis the hydrogen reaction is fastest. CATALYZING THE HYDROGEN AND OXYGEN EVOLUTION REACTIONSThese reactions are all happening at the surface of the electrode, and the equations are not reflecting that as they stand. That is because these are electron transfer reactions, and the electrons flow from the cathode and into the anode. So, the anode and cathode have to be conductive, generally metals are used but can more exotic forms of carbon may also be promising. How easy those reactions happen (i.e. how fast they occur), depends on the morphology and chemical composition of the surface of the electrode. Here other catalysts are important … what catalysts are used depends on whether water electrolysis is acid or base catalyzed. Since not all catalysts work or are stable in an acidic or basic environment.
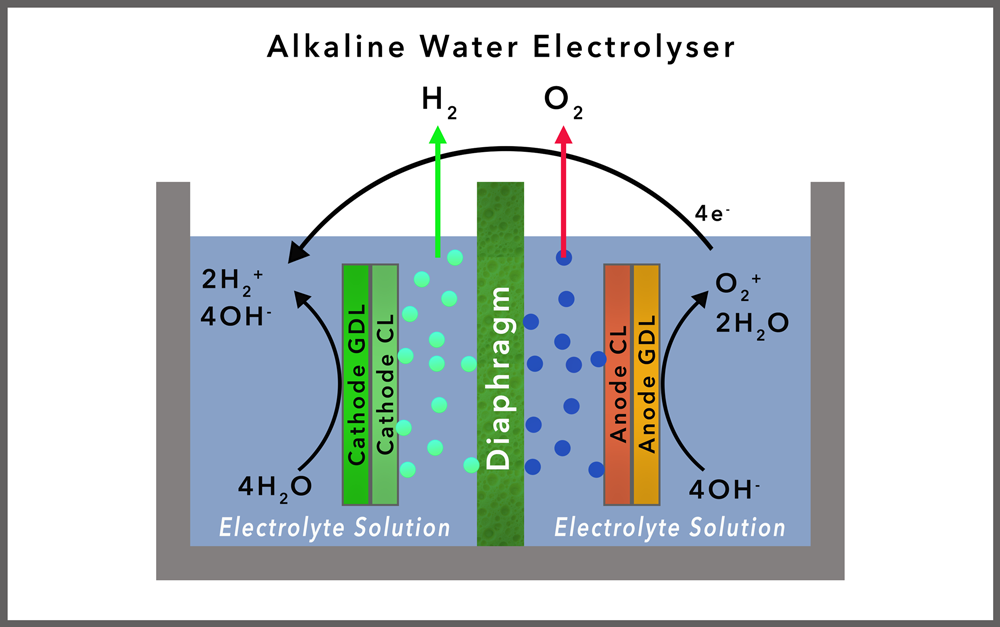
THE NEED FOR A SEPARATORTo help with the separation of the hydrogen from the oxygen the anode and cathode are separated from one another by a semipermeable separator (membrane) that facilitates passage of water and ions but prevents the cross-over of the gases from one compartment to the other. The ideal separator needs to be hydrophilic, resistant to corrosion, durable, thermally stable, permeable to ions, while preventing gases crossing from one side to the other, and inexpensive.
In most commercial applications, the separators are comprised of long-chain polymers that, on aggregating, create films with a microporous channels through which ions and water can diffuse/migrate. These are referred to as micro-porous separators.
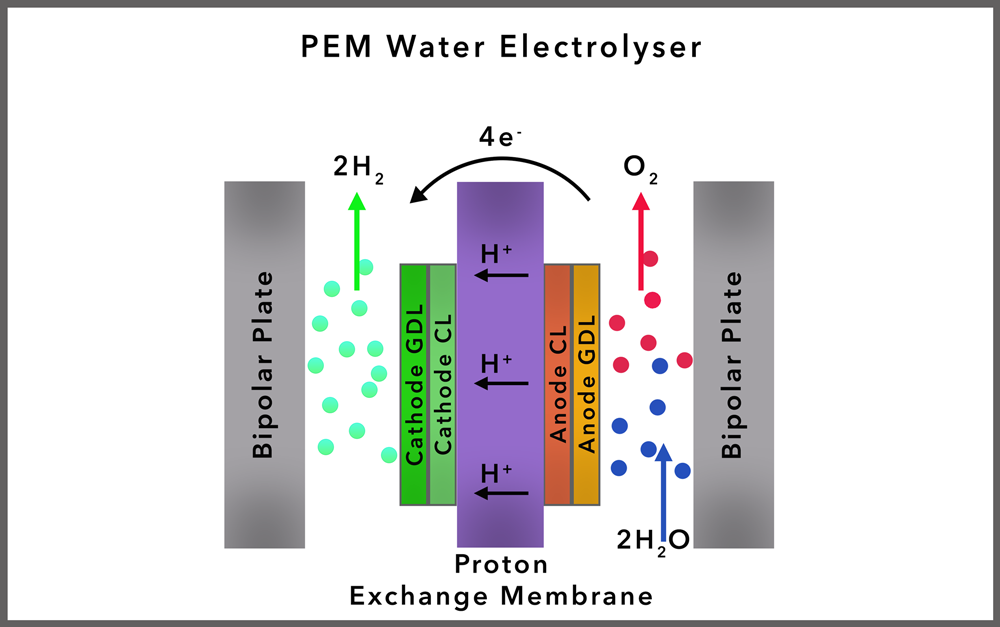
Sometimes special membranes are used that allow only water and hydrogen ions to pass through, these are called Proton Exchange Membranes (PEMs) these are used in acidically catalyzed water electrolysis. The membrane has to be stable in acid, which is highly corrosive, currently long chain poly fluorinated sulphonic acids are used. Commercially this is available as Nafion® made by Dupont.
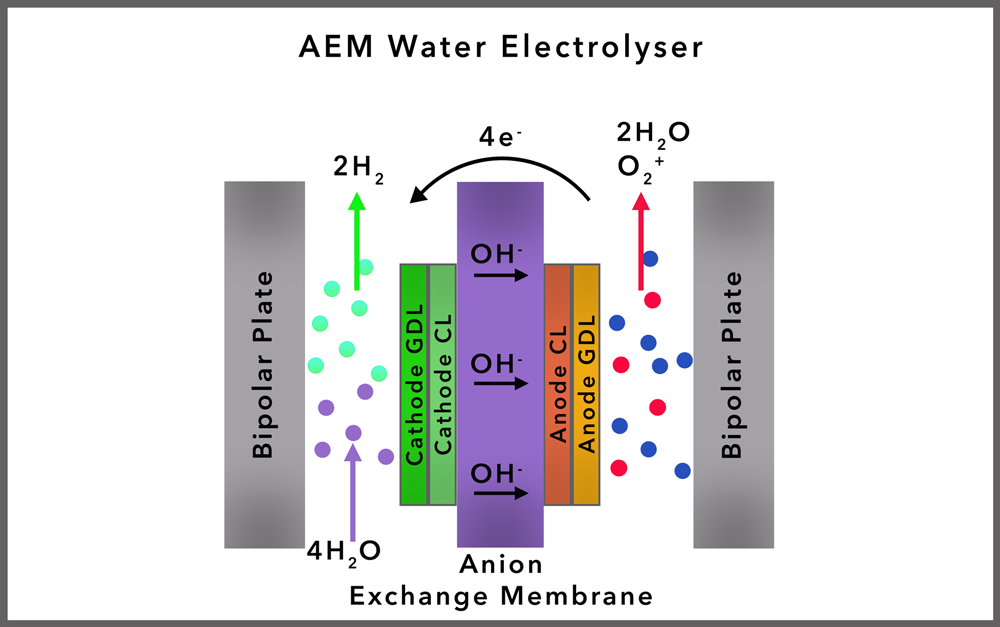
There is an equivalent for alkaline catalyzed water electrolysis where anions and water can permeate through the membrane, these are called Anion Exchange Membranes (AEMs). To our knowledge no such electrolyzer is commercially available yet. For alkaline water electrolysis (AWE) these membranes need to be resistant to corrosion in a basic environment, as hydroxide solutions are also corrosive.
Alternatively membranes used can be ambivalent, being hydrophilic, and permeable to both anions and cations. They need to be stable in the environment in which which they are used, be it acidic or alkaline. Polyether Sulphones (PES) and polysulfones (PSU) have promise in this area. AGFA Zirfon® Perl separators are a hybrid of these two membranes along with polyphenylene sulfide (PPS) with encapsulated ZrO2 particles to increase the hydrophilicity of the membrane. This membrane is widely used in commercial AWE because of its longevity. Sulphonated polyether ether ketone (sPEEK) is a membrane that may be suited to both PEM and alkaline systems.
Historically older AWEs use asbestos separators, an inorganic magnesium silicate, before asbestos got banned in most countries. This is an example of a crystalline inorganic material. This is more resistant to both acidic and alkaline water electrolysis and more thermally stable than its polymeric counterparts.
MEMBRANES DEGRADE OVER TIMEAsbestos aside, polymeric membranes are perhaps the most fragile part of most commercial electrolyzers. They have a finite life, so while they are resistant to corrosion they still corrode, that gets accentuated at high temperatures where corrosion becomes more of a problem. So Zirfon® is stable up to 80oC, Nafion® … which is related to Teflon …. Can function up to 100oC, but can become clogged by errant metal ions, which can appear due to water contamination or the natural disintegration of the anode over time. Membranes must get swapped out periodically … to maintain the optimal performance of the electrolyzer.
ZERO GAP ELECTROLYZERSZero gap electrolyzers have the anode and cathode sandwiching the separator membrane … this minimizes the distance the ions travel … on their path from one electrode to the other. They just must pass through the separator. This reduces the ion resistance. This helps with energy efficiency at high currents, so in principle zero gap designs are more efficient.
As the global demand for clean energy grows, several electrolysis technologies have emerged, each with its advantages and challenges. This section explores the five main types of hydrogen electrolysis technologies highlighting their pros, cons, costs, market deployment, and life expectancy:
PROTON EXCHANGE MEMBRANE (PEM) ELECTROLYSIS
Overview: PEM electrolysis uses a solid polymer electrolyte (the proton exchange membrane) that conducts protons from the anode to the cathode, while separating the hydrogen and oxygen gases produced. Cost: $1,200 - $2,000/kW Hydrogen Purity: 99.99%
Pros:
Cons:
Cost: PEM electrolysers are more expensive than alkaline systems, with costs around $1,200 to $2,000 per kilowatt, though prices are expected to decrease with advancements in catalyst development and manufacturing processes (as are other electrolyser technologies).
Market Deployment: PEM technology is well established, particularly in small to medium-scale applications, such as hydrogen fueling stations and backup power systems. The global PEM electrolyser market is growing rapidly, driven by the increasing adoption of green hydrogen.
Life Expectancy: The lifespan of PEM electrolysers is typically touted as 50,000 to 80,000 operational hours, though actual performance can vary based on operating conditions with some industrial facilities powered by renewables demonstrating an operational life as low as 20,000 hours. Ongoing research is focused on extending membrane life and reducing reliance on PGMs.
ANION EXCHANGE MEMBRANE (AEM) ELECTROLYSIS
Overview: AEM electrolysis is an emerging technology that uses an anion exchange membrane to transport hydroxide ions from the cathode to the anode, facilitating the electrolysis process. The AEM operates using a liquid alkaline electrolyte (typically potassium hydroxide) to conduct ions between two electrodes, similar to Alkaline electrolysers.
Cost: $800/kW Hydrogen Purity: 99.99%
Pros:
Cons:
Cost: AEM electrolysers are expected to be less expensive than PEM systems once they reach commercial maturity.
Market Deployment: AEM technology is not yet widely deployed and is primarily in the research and pilot project phase. However, it holds significant potential for cost reduction and wider adoption in the future.
Life Expectancy: The life expectancy of AEM electrolysers is to be determined, with ongoing research focused on improving membrane durability and performance. Initial development suggests a life in line with PEM.
ALKALINE ELECTROLYSIS
Overview: Alkaline electrolysis is the most established electrolysis technology, using a liquid alkaline electrolyte (typically potassium hydroxide) to conduct ions between two electrodes. Alkaline tend to be bipolar electrolysers.
Cost: $500 - $1,000/kW Hydrogen Purity: 99.5% - 99.9%
Pros:
Cons:
Cost: Alkaline electrolysers are among the cheapest, with costs ranging from $500 to $1,000 per kilowatt. Their long history of use has led to cost reductions through economies of scale.
Market Deployment: Alkaline technology dominates large-scale industrial hydrogen production, particularly in applications where space is less of a constraint. It remains the most widely deployed electrolysis technology globally.
Life Expectancy: Alkaline electrolysers have a long lifespan, often exceeding 100,000 operational hours. However, maintenance can be more frequent due to the corrosive nature of the alkaline electrolyte.
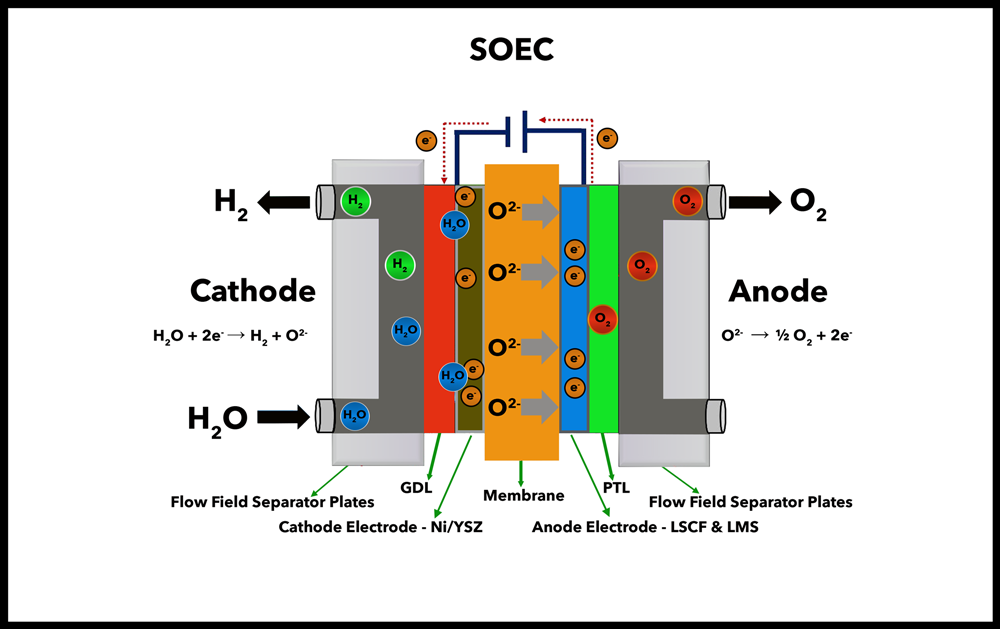
SOLID OXIDE ELECTROLYSIS (SOEC)
Overview: Solid Oxide Electrolysis Cells (SOECs) operate at high temperatures (around 700-1,000°C) and use a solid ceramic electrolyte to produce hydrogen. This high-temperature operation enables higher efficiencies of conversion.
Cost: $2,500 - $5,000/kW Hydrogen Purity: 99.99%
Pros:
Cons:
Cost: SOEC technology is still in the early stages of commercialization, with costs ranging from $2,500 to $5,000 per kilowatt. These costs are expected to decrease as the technology matures and production scales up.
Market Deployment: SOECs are primarily in the demonstration and pilot phase, with a focus on industrial applications where high-temperature waste heat is available.
Life Expectancy: The lifespan of SOEC systems is currently shorter than other technologies due to the high operating temperatures, with most systems achieving around 40,000 to 60,000 operational hours. Research is ongoing to improve the durability of SOEC materials.
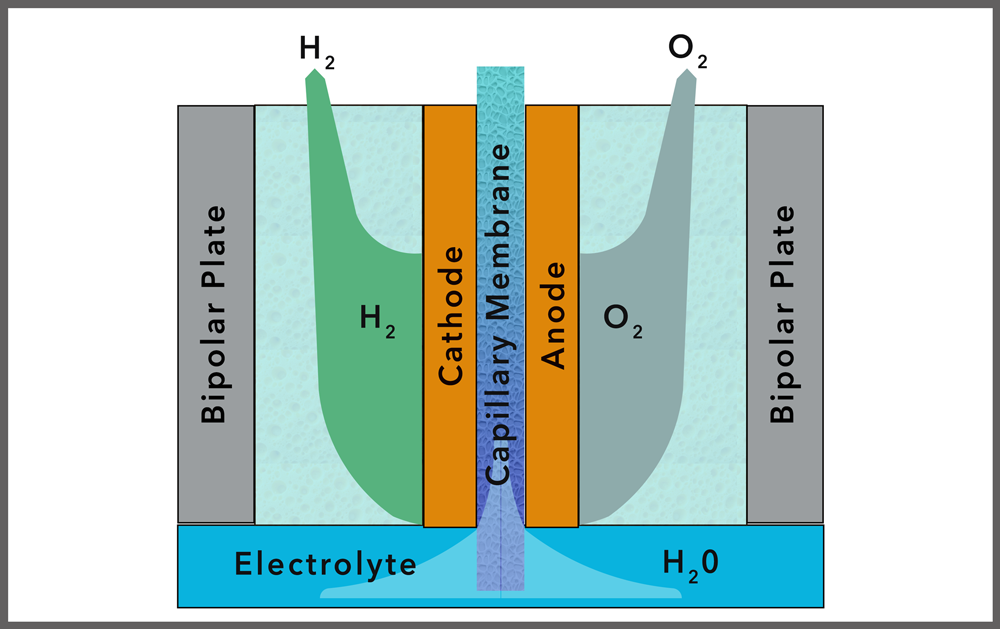
CAPILLARY ACTION ELECTROLYSER (ZERO-BUBBLE)
Overview: The capillary action electrolyser, also known as the no-bubble electrolyser, is an innovative technology that is currently under development. This type of electrolyser design aims to overcome some of the key limitations of traditional electrolysis technologies by employing a different approach to water management and gas production.
Traditional electrolysis involves passing an electric current through water, splitting it into hydrogen and oxygen gases, which are then collected at the respective electrodes. However, during this process, bubbles of hydrogen and oxygen form on the electrodes, which can reduce the efficiency of the reaction by creating resistance and blocking the electrode surface.
The capillary action electrolyser addresses this issue by using a capillary action mechanism to manage the flow of water and the removal of gases. In this design, water is drawn into the electrolyte channels via capillary action. The gas bubbles produced during the electrolysis are immediately removed from the electrode surface through the same capillary channels, preventing the formation of large bubbles that hinder the electrolysis process.
The process reduces the electrical resistance of the electrolysis created by the bubbles to improve efficiency but does potentially introduce issues not associated with alternative electrolyser technologies.
Cost: $ To be determind Hydrogen Purity: 99.99%
Pros: Increased Efficiency: By preventing the formation of large gas bubbles, the capillary action electrolyser reduces the resistance at the electrodes, leading to higher overall efficiency. This could result in lower energy consumption per kilogram of hydrogen produced.
Lower Operating Costs: The increased efficiency and reduced energy consumption can translate into lower operating costs, making green hydrogen production more economically viable.
Cons: Technological Maturity: As an emerging technology, the capillary action electrolyser is still in the development and testing phases. It has yet to be widely adopted or proven at a large commercial scale, which may pose risks for early adopters.
Industrialisation Hurdles: There are potential issues around the size of the electrolyser due to the nature and laws surrounding a capillary action. Commercialisation of an industrial scale Capillary Action electrolyser are yet to be demonstrated.
Initial Investment Costs: While the operational costs may be lower, the initial capital investment for deploying this new technology could be higher compared to more established electrolysis methods, depending on the scale and application.
Cost: Capillary Action Electrolysers are still in the infancy of development, so production and operational costs are hard to evaluate. In theory the efficiency will be very good so the electrical cost relating to hydrogen generation should be lower than the alternative technologies.
Market Deployment: Gaining market acceptance and trust in a new technology can be challenging, especially when competing against well-established electrolysis technologies like PEM and alkaline electrolysers. The adoption rate to the market could be slow.
Life Expectancy: Capillary Action electrolysers should have an increased life expectancy, though they operate with an alkaline electrolyte like an Alkaline electrolyser, and this can be highly corrosive.
CONCLUSION Each hydrogen electrolysis technology has its unique advantages and challenges. PEM is favoured for its high efficiency and flexibility but is hindered by high costs due to reliance on platinum group metals. AEM offers potential cost reductions but is still in the early stages of development. Alkaline electrolysis remains the most cost-effective and widely deployed, though it lags in efficiency and responsiveness. SOECs promise high efficiency but face significant materials challenges due to high operating temperatures. Capillary Action is too early in the development curve to be judged.
As the global demand for green hydrogen grows, the choice of electrolysis technology will depend on specific application needs, cost considerations, and the availability of renewable energy. Ongoing research and development are critical to overcoming the current limitations and unlocking the full potential of these technologies for a sustainable energy future.
Sources
|