AMMONIA (NH₃): PROPERTIES, PRODUCTION PROCESSES, AND ENVIRONMENTAL IMPACT
Ammonia (NH₃) is a fundamental chemical with a wide array of applications across multiple industries. Its primary use is in the production of nitrogen-based fertilizers, which account for approximately 80-85% of global ammonia production. This industry is pivotal, with the global market for nitrogen fertilizers valued at over $70 billion, closely tied to worldwide agriculture and food production.
However, ammonia's applications extend beyond agriculture, playing vital roles in chemical manufacturing, refrigeration, and, more recently, as a potential hydrogen carrier in clean energy transitions.
PRODUCTION PROCESSES OF AMMONIA
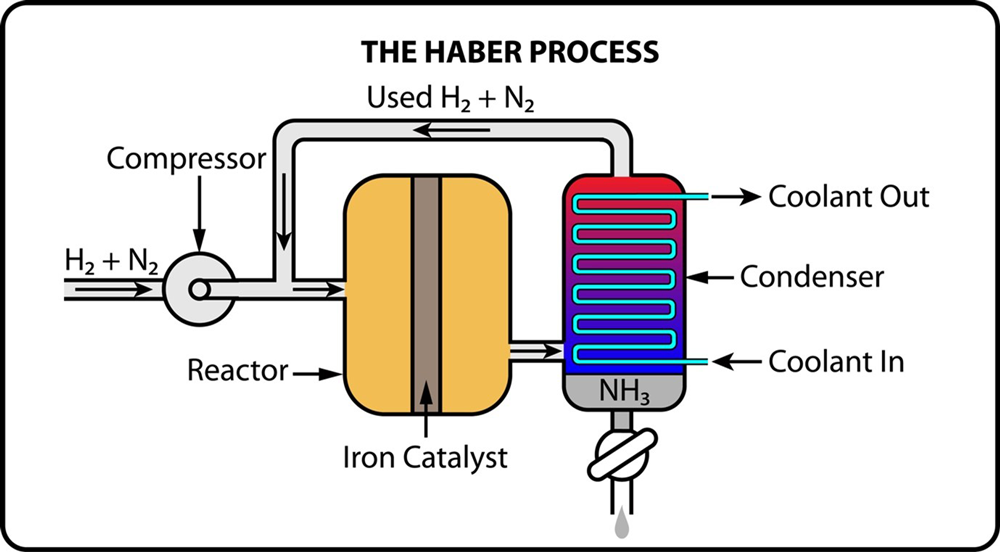
The Haber-Bosch process is the predominant method for producing ammonia, responsible for nearly all global ammonia production, totalling around 180 million tons annually. This process synthesizes ammonia by combining nitrogen (N₂) from the air with hydrogen (H₂) under high pressure (150-250 bar) and temperature (400-500°C) in the presence of an iron catalyst.
Green ammonia is an emerging alternative that addresses the environmental concerns of traditional production methods. Green ammonia is produced using hydrogen derived from water electrolysis powered by renewable energy sources such as wind, solar, or hydroelectric power.
KEY COMPARISONS BETWEEN AMMONIA AND GREEN AMMONIA
GREY V GREEN HYDROGEN AMMONIA – CONSUMPTION & EMISSIONS
Energy Requirements
To determine the energy used in producing 1,000 kg of ammonia (NH₃) when green hydrogen is used instead of grey hydrogen, we calculate the consumption in the following way.
Step 1: Hydrogen Requirement for Ammonia Production
For 1,000 kg of ammonia:
Hydrogen required = 1,000 kg × 0.1765 = 176.5 kg of H2
Step 2: Energy Required to Produce Green Hydrogen (HFI)
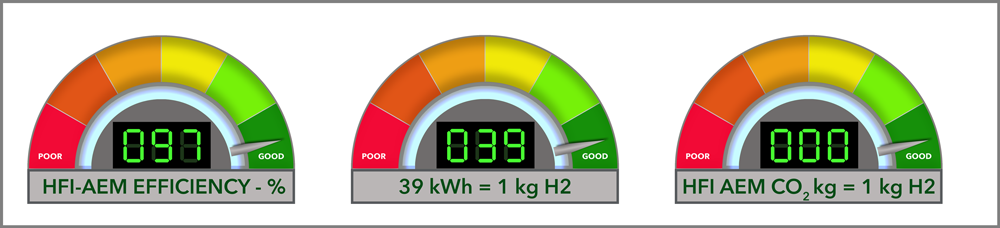
Producing green hydrogen via electrolysis typically requires about 50-55 kWh per kg of H₂. The HFI hydrogen production system requires significantly less energy at approximately 40 kWh/kg, and this is what we will use for this calculation.
Energy for 176.5 kg of H₂= 176.5 kg × 40 kWh/kg = 7,060 kWh
Step 3: Grey Hydrogen Energy Consumption (Baseline)
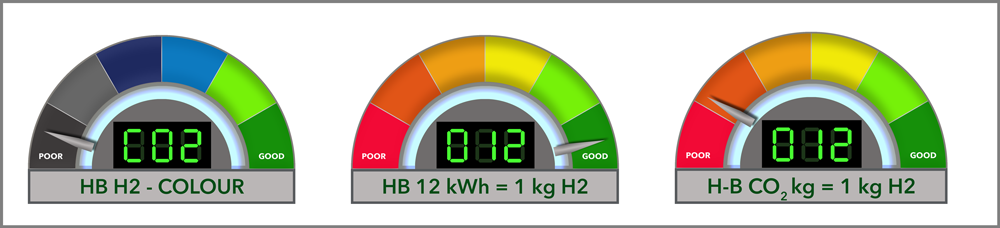
Producing grey hydrogen via steam methane reforming (SMR) typically requires about 8-12 kWh per kg of H₂.
Energy for 176.5 kg of H₂ = 176.5 kg × 12 kWh/kg = 2,118 kWh
Step 4: Difference Between Green (HFI) and Grey Hydrogen
The additional energy required when using green hydrogen instead of grey hydrogen:
ΔEnergy = 7,060 kWh – 2,118 kWh = 4,942 kWh
When using green hydrogen instead of grey hydrogen, producing 1,000 kg of ammoniarequires approximately 4,942 kWh more energy.
CO2 EMISSIONS
Emissions for Grey Hydrogen Based Ammonia
Grey hydrogen is typically produced via steam methane reforming (SMR). The process emits approximately 9-10 kg of CO₂ per kg of H₂.
Using an average of 9.5 kg of CO₂ per kg of H₂:
= 176.5 kg of H₂ × 9.5 kg CO₂/kg H₂
CO₂ emissions = 1,676 kg of CO₂
CO₂ Emissions for Green Hydrogen
Green hydrogen is produced using renewable energy (via electrolysis). It is carbon-free, so:
CO₂ emissions from green H₂ production = 0 kg CO₂.
Additional CO₂ Emissions in Ammonia Production
The production of ammonia involves additional steps, but the CO₂ emissions difference primarily arises from the hydrogen source.
Assuming no significant differences in downstream emissions:
CO₂ emissions difference = 1,676 kg CO₂
The difference in CO₂ emissions between green ammonia and grey ammonia for 1,000 kg of ammonia is approximately 1,676 kg of CO₂, with green ammonia being the carbon-free option.
So, while considerably more energy is required to produce ammonia cleanly the reduction in emissions is very significant. You pay your money, and you make your choice.
AMMONIA AS A HYDROGEN CARRIER
Ammonia is increasingly recognised as an efficient hydrogen carrier due to its high hydrogen content (17.8% by weight). It can be liquefied at relatively low pressures (8-10 bar), making it easier and more cost-effective to store and transport compared to liquid hydrogen, which requires cryogenic temperatures.
HYDROGEN EXTRACTION FROM AMMONIA
The extraction of hydrogen from ammonia is a critical step in using ammonia as a hydrogen carrier. Here are the main methods:
THERMAL CRACKING (DECOMPOSITION)
CATALYTIC CRACKING
ELECTROCHEMICAL AMMONIA SPLITTING
PLASMA-ASSISTED AND PHOTOCATALYTIC DECOMPOSITION
Ammonia is a vital chemical with significant industrial applications, but its traditional production methods are a major source of CO₂ emissions. Green ammonia and advanced hydrogen extraction technologies offer promising pathways to reduce the environmental impact of ammonia production. However, challenges remain, particularly in terms of energy efficiency, safety, and cost. Ongoing research and development are crucial to overcoming these challenges and realizing the full potential of ammonia as a sustainable hydrogen carrier.
|